Giacomo Pirovano, Sheryl Roberts, Thomas Reiner*
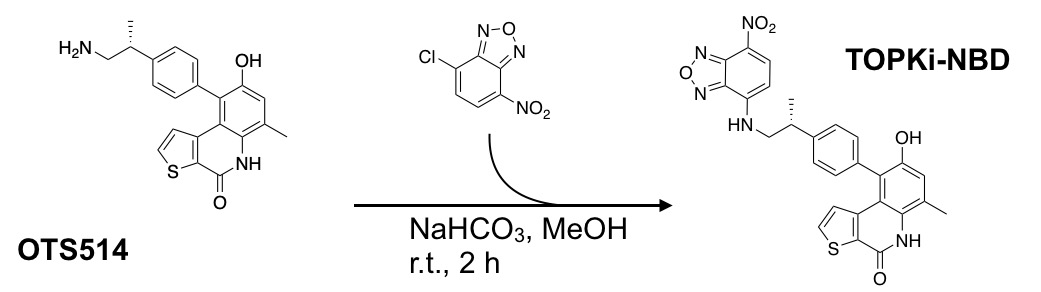
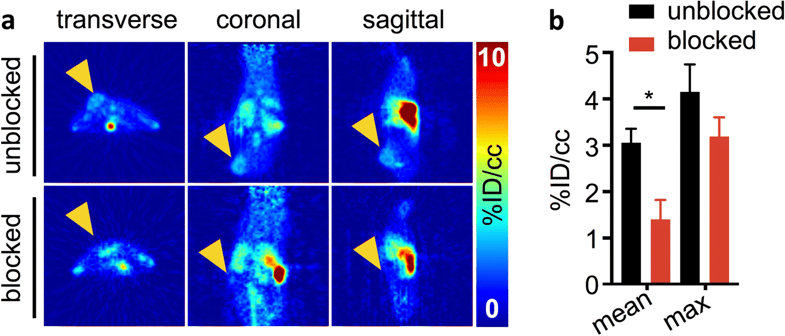
Purpose: OTS514 is a highly specific inhibitor targeting lymphokine-activated killer T cell-originated protein kinase (TOPK). A fluorescently labeled TOPK inhibitor could be used for tumor delineation or intraoperative imaging, potentially improving patient care. Methods: Fluorescently labeled OTS514 was obtained by conjugating the fluorescent small molecule NBD to the TOPK inhibitor. HCT116 colorectal cancer cells were used to generate tumors in NSG mice for in vivo studies. Images were generated in vitro using confocal microscopy and ex vivo using an IVIS Spectrum. Results: OTS514 was successfully conjugated to a fluorescent sensor and validated in vitro, in vivo, and ex vivo. The labeling reaction led to TOPKi-NBD with 67% yield and 97% purity after purification. We were able to test binding properties of TOPKi-NBD to its target, TOPK, and compared them to the precursor inhibitor. EC50s showed similar target affinities for TOPKi-NBD and the unlabeled OTS514. TOPKi-NBD showed specific tumor uptake after systemic administration and was microscopically detectable inside cancer cells ex vivo. Blocking controls performed with an excess of the unlabeled OTS514 confirmed specificity of the compound. Overall, the results represent a first step toward the development of a class of TOPK-specific fluorescent inhibitors for in vivo imaging and tumor delineation. Conclusions: TOPK has the potential to be a new molecular target for cancer-specific imaging in a large variety of tumors. This could lead to broad applications in vitro and in vivo.